 By: Rachel Lau, Social Media & Marketing Associate, Guardian Data Destruction IT departments and risk management teams are often unsure what to do with highly sensitive or data-laden equipment. Especially when that hardware is old and has no real market value. When they don’t know, the default choice is usually to do nothing. Unused laptops, desktops, USB drives, servers, printers, phones and other smart devices eventually move to a drawer then a closet where they’re dust covered and forgotten. More often, end-of-life IT equipment is collected and locked up in a storage unit or basement, successfully converting it to a “forever” inventory problem. The delay (or denial) in IT asset disposition decision-making has significant short term and long-term risks:
At Guardian, we’re accustomed to working with companies that don’t know how to reduce and eliminate embedded data on stored end-of-life IT equipment. What happens when we walk through a storage unit or warehouse full of old computers? You see technology dinosaurs and we see BIG risk. No matter what range of devices you find, we work with ITADs, VARs and resellers to develop the data destruction and disposition solution that makes even your most difficult stakeholder satisfied. Don’t investigate storage facilities or other iffy solutions. Talk to us about a strategy to safely destroy the data and erase your risk. A comprehensive data destruction plan will prudently empty your IT closet or warehouse without any chance of a data breach or long term, ongoing repository and expense.
0 Comments
 By: Geoffrey R. Kaiser, Senior Counsel, Rivkin Radler The Office of Inspector General, Department of Health and Human Services (OIG) has finalized new safe harbors and modifications of existing safe harbors under the federal Anti-Kickback Statute (AKS) to reflect a policy priority favoring a value-based health care system that “pays for health and outcomes” and that will “remove potential barriers to more effective coordination and management of patient care and delivery of value-based care.” In total, there are seven new safe harbors and four modifications to existing safe harbors. Each has its own elements that must be satisfied for the arrangement to be protected. The Final Rule becomes effective January 19, 2021. Certain entities, deemed by OIG not to be on the front lines of care coordination and that pose a higher risk of fraud or abuse, are ineligible to use certain new safe harbors for value-based arrangements, outcomes-based payments and patient engagement. These entities include pharmaceutical and device manufacturers, compounding pharmacies and DME suppliers; however, there is an exception, in the case of device and DME companies, for care coordination arrangements involving in-kind remuneration consisting of digital health technology. Even ineligible entities, however, may be able to use other new or modified safe harbors. The Final Rule also codifies an exception to the prohibition of beneficiary inducements under the Civil Monetary Penalties Law (CMPL) for telehealth technologies furnished to certain in-home dialysis patients, and notes that by operation of law any arrangements that fit in the new and modified AKS safe harbors for patient engagement and support and local transportation are also protected under that CMPL provision. The new AKS safe harbors and modifications are: Value-Based Arrangements These safe harbors cover remuneration exchanged between or among participants in a value-based arrangement that fosters better coordinated and managed patient care, including:
These safe harbors are intended to provide “flexibility for innovation and customization of value-based arrangements to the size, resources, needs, and goals of the parties to them,” including “emerging arrangements that reflect up-to-date understanding in medicine, science, and technology.” All three safe harbors provide protection for in-kind remuneration, but only the two safe harbors with substantial assumption of risk protect monetary remuneration. OIG noted, however, that “parties to arrangements involving monetary remuneration, such as shared savings or performance bonus payments, may be eligible for the new protection for outcomes-based payments at [§1001.952(d)(2)]” and “parties to arrangements under CMS-sponsored models may prefer to look to the new safe harbor specifically for those models [§1001.952(ii)].” Patient Engagement and Support This safe harbor permits certain tools and supports furnished by a participant in a value-based enterprise to patients to improve quality, health outcomes and efficiency [§1001.952(hh)]. As with the value-based safe harbors, this safe harbor is not available to the same group of ineligible entities, except that a device manufacturer may provide a patient engagement tool or support that consists of digital health technology. CMS-Sponsored Models This safe harbor protects remuneration provided in connection with certain CMS-sponsored models (i.e., Medicare shared savings program or other model being tested by CMS under 42 USC 1315a(b)) [§1001.952(ii)]. This safe harbor is “intended to provide greater predictability for model participants and uniformity across models” and “will reduce the need for separate OIG fraud and abuse waivers for new CMS-sponsored models.” Cybersecurity Technology and Services This safe harbor protects donations of cybersecurity technology and services. It is intended to “facilitate improved cybersecurity in health care and is available to all types of individuals and entities.” [§1001.952(jj)] Electronic Health Records Safe Harbor The existing safe harbor for electronic health records items and services has been modified to “update and remove provisions regarding interoperability, remove the sunset provision and prohibition on donation of equivalent technology, and clarify protections for cybersecurity technology and services included in and electronic health records arrangement.” [§1001.952(y)] Personal Services and Management Contracts and Outcomes-Based Payments The existing safe harbor for personal services and management contracts has been modified “to increase flexibility for part-time or sporadic arrangements and arrangements for which aggregate compensation is not known in advance.” The safe harbor also adds new protection “for certain outcomes-based payments and part-time arrangements.” [§1001.952(d)]. The added protection is “tied to achieving measurable outcomes that improve patient or population health or appropriately reduce payor costs.” The same ineligible entities list applies to this safe harbor. Warranties The existing safe harbor for warranties has been modified to revise the definition of “warranty” and provide protection for bundled warranties for one or more items and related services. OIG noted that the safe harbor “could extend to arrangements conditioned on clinical outcomes guarantees, which could include warranties conditioned upon ‘value-based’ outcomes that meet the safe harbor’s other requirements.” This safe harbor is available to all types of entities. [§1001.952(g)] Local Transportation The existing safe harbor for local transportation has been modified to expand mileage limits for rural areas (up to 75 miles) and “eliminate mileage limits for transportation to convey patients discharged from the hospital to their place of residence” when discharged from an inpatient facility or released from a hospital after being placed on observation for at least 24 hours. The safe harbor also clarifies that it is available for transportation provided through rideshare arrangements. [§1001.952(bb)] ACO Beneficiary Incentives The Final Rule also codifies the statutory exception to the definition of remuneration at 42 USC §1320a-7b(b)(3)(K) related to ACO Beneficiary Incentive Programs for the Medicare Shared Savings Program. [§1001.952(kk)] 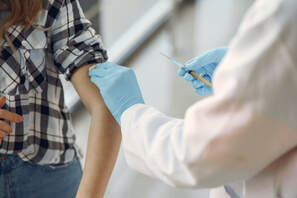 By: Brigette N. Eagan, Counsel, Genova Burns We have seen recent articles speculating on whether employers can require their employees to take an FDA-approved COVID-19 vaccination as a condition of employment or continued employment. Here, without giving any legal advice, for private (non-governmental) New Jersey employers, odds are that this type of employment rule will be permissible, with certain limitations, discussed below. Predicting the permissibility of a COVID-19 vaccination program is an easier question for New Jersey employers to answer. This is because, this year, the State of New Jersey required employers that are hospitals, nursing homes, and home health care agencies to adopt plans mandating that their employees take the influenza vaccine. The requirement applies to those employees with direct patient contact as well as “back-room” and administrative employees. If the State of New Jersey now requires certain employers to implement mandatory influenza vaccination programs (even for employees with no involvement in patient care), it is not a far reach that, given the physical, mental, and financial loss caused by COVID-19, our legislature and courts will find mandatory COVID-19 programs permissible, as long as those programs comply with other federal and state employment laws. EXEMPTIONS TO MANDATORY VACCINATIONS The New Jersey influenza vaccination law contains a medical exemption. Specifically, employees can request an exemption from the influenza vaccination requirement if they can show a medical contra-indication to the vaccination (this means generally that employees can be excused from the vaccination requirement dependent upon some serious medical consequence to taking the vaccine). The New Jersey vaccination law, however, does not contain an express exemption for employees who object to the vaccine based upon their religious beliefs. It has not been tested yet, as to whether our New Jersey courts will read an obligation into the law for employers to accommodate employees’ religious objections to the influenza vaccination. Where does the New Jersey influenza vaccination law leave us in terms of predicting the permissibility of employer-required COVID-19 programs? We are entering new ground when we think about employers requiring vaccinations as a condition of employment. We have also entered a new world, where fatalities exceed 250,000 individuals in the Unites States, and where unvaccinated employees can be seen as a direct threat to their co-worker’s safety. Drastic times, drastic measures. Employers considering COVID-19 vaccination programs should still tread lightly. Again, without providing any legal advice, consider including exemptions for medical issues and religious beliefs, in order to defend against challenges to applicable employment laws, such as the New Jersey Law Against Discrimination (which covers religious and medical issues), Title VII of the Civil Rights Act (for religious beliefs), and the Americans With Disabilities Act (for medical issues). As to employees who fall within these exemptions, what accommodations will you, the employer, consider? The glaring accommodation is to allow the employee to work remotely, if the job allows it. If no accommodations are possible, will your policy include unpaid leave or will termination be the only option? EMPLOYEES WHO OBJECT TO MANDATORY VACCINATIONS AND EMPLOYEE EDUCATION There are many other issues employers must plan for when implementing COVID-19 vaccination programs. Employees may claim that they do not trust the vaccine; they worry that they may become ill from it. Or, they may claim the vaccine is simply a political issue, and their objections to the vaccine amount to job-protected whistle-blowing. Others will say that a mandated vaccine violates rights to freedom over their body and speech. How will these employees be treated? In order to defend against some of these types of claims, employers may opt to roll out training simultaneously with their COVID-19 vaccination program (similar to common anti-discrimination training used to educate employees). Or, will your policy be a little more aggressive, and provide that the COVID-19 vaccination is an essential job requirement and failure to comply will result in immediate termination? OTHER PRACTICAL CONSIDERATIONS Once your policy is in place, how practically, will you roll it out? Will you have an on-site vaccination program, similar to the flu vaccination where a pharmacist or technician administers the vaccine during the work day? Will you pay for the vaccine? Or, before allowing employees to enter the facility, will you require them to be vaccinated off-site? Again, who will pay the cost, especially for those employees without employer-provided insurance? BOTTOM LINE As this article demonstrates, there are more questions than answers at this point. There is hope that the vaccine may be available later this year or early next, on a limited basis. Now is the time to start asking these questions, gauging your own comfort level as the employer, and contemplating what your policies will look like and then asking if they comply with the law. This is an area where we must think hard, but at the same time be comprehensive and firm within the confines of the law. Genova Burns is here to help you traverse this road. For guidance navigating COVID-19 issues at your workplace, please contact Dina M. Mastellone, Esq., Chair of the firm’s Human Resources Counseling & Compliance Practice Group, via email here or 973.533.0777, or Brigette N. Eagan, Esq., Counsel in the firm’s Human Resources Counseling & Compliance Practice Group via email here or 973.533.0777. |
Guest Blog
Archives
July 2024
Categories |
- About Us
- Events & Programs
-
Forums
- Diversity, Equity & Inclusion Forum
- Environmental Business Council
- Family-Owned Business Forum
- Healthcare Foum
- Higher Education Forum
- Human Resources Forum
- International Forum
- Manufacturing Forum
- Marketing Forum
- Next Gen: A Young Professionals Organization
- Non-Profit Forum
- Real Property Forum
- Technology for Business Forum
- Transportation Forum
- Women in Commerce Forum
- Membership
- COMMERCE Magazine
- Member Directory
- Member News
- Guest Blog
|
Copyright Commerce and Industry Association of New Jersey. All Rights Reserved.
365 West Passaic Street Suite 490 | Rochelle Park, NJ 07662 Phone: (201) 368-2100| [email protected] | sitemap |

 RSS Feed
RSS Feed
